One of the key technological steps for our work in the lab is "constructing" yeast strains that carry combination of genetic perturbations. In our first try to perform such steps in a high-throughput manner, Avital had very nice results.
Some background first. An important approach to study organism is to modify some genes and see the effect, and from that deduce something about the function of the gene. One of the reasons yeast is such a great model organism for research is that performing genetic modifications --- replacing one piece of DNA with another --- is relatively easy.
Nonetheless, creating many combinations of such modifications one by one would be tedious. Here people in the yeast biology invented clever tricks to do so easily. The idea is simple and is based on simple Mendelian genetics. In nutshell, suppose we have one yeast strain with modification X and one with modification Y. If we can "marry" them and have them generate offsprings, than 1/4 of them would inherit both the X and the Y modification. If we somehow could select these specific offsprings we are managed to combine the two modifications.
This description is oversimplified in several ways. It assumes that X and Y are "unlinked" in genetics terms, that is, the inheritance of X does not influence the inheritance of Y. In practical terms this means that X and Y are either on separate chromosomes, or sufficiently far to allow for recombination (in which case the fraction of offsprings with both X and Y gets smaller as X and Y are closer).
Secondly, we do not marry yeasts. Instead, we use the fact that the yeast lives in two forms. A haploid yeast has one copy of each chromosome, and a diploid yeast has two copies. (In case you wonder, all of our cells are diploid, except for cells involved in reproduction.) The yeast prefers to live as a diploid, and thus two haploid yeasts with different mating type (the yeast version of gender) will fuse together to form a diploid cell. However, when the times get tough, a diploid yeast will undergo meiosis and divide into four haploid spores, which are very resistant and will survive until things get better.
The yeast geneticists use this behavior to create combinations of perturbation. Basically the idea is to go through the stages shown here
This method uses antibiotic markers (NAT and KAN here) to select only the spores that have inherited both mutations (shown as filled ovals here). The idea, initially pushed by Charlie Boone from Toronto, is that this process can be carried in parallel for many yeast strains. We do so by growing yeast on agar plates, each with the right combination of nutrients and antibiotic to select the cells that we want from the previous stage in the above diagram. All these steps relay on our Singer RoToR robot for copying colonies from one plate to the next.
The weeks before Passover, Avital run our first mating protocol. She created a yeast with a knockout (KO) of the Gal1 gene, and then mated that yeast with plate of 96 strains from the Yeast Knock-Out library that is supposed to contain all viable knockout strains.
The original knockout plate she choose was one that contains Gal1 KO as well as KO of few genes that are adjacent to Gal1 on yeast Chromosome 2. The original plate we got didn't have all the strains, which is often the case as KOs are very sick and do not propogate.
Avital mated these with the Gal1 KO she created, and at the same time copied the original 96-colony format to 384-colonies, so that each mating experiment was propagated four independent times. The diploid plate looks like the original plate, except each colony was replaced by four smaller colonies.
She then copied the cells onto a plate with "sporulation media" - poor media that drives diploid yeast to sporulate. Since the media is poor, the colonies do not grow much from the original amount deposited by the pinning.
Once the colonies are copied to fresh rich media they grow again, but now only ones that carry the original KO (as we selected for it).
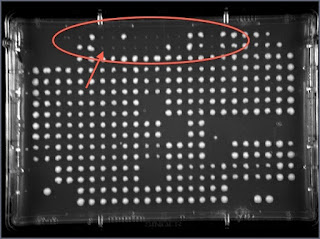
Once Avital moved cells to a plate that also selects for the second KO, some colonies disappeared (circled in red).
You can see a bit of the original copied cells that did not manage to grow. The quartet where all colonies are missing (red arrow) are exactly the Gal1 KO --- since both KOs are in the same genomic location we can create a strains that contain both of them. In nearby regions we get few successes (unlikely event) but not consistently. Elsewhere, we get the double KO colonies.
You might notice that we also lost colonies in the corner (e.g., lower left corner) but this is probably due to uneven agar levels --- something we need to fine tune.





No comments:
Post a Comment